Classify Each Compound Below as Ionic or Covalent
A C B N C O D F. Drag the appropriate compounds to their respective bins.

Solved Practice Problem 01 45 In The Compounds Below Chegg Com
Copper II sulfide ionic or covalent ion is 2 is cobalt Sulfate ionic or covalent bond below.
. Classify the following compounds as ionic or covalent. IonicCovalent Covalent Type Name ionic Nigneldibremmide NiBr2 onic Tetracarbon decanydrogen CAH10 Coalent Cacboo aisuifide CS. If covalent classify them as binary hydrocarbons or acids.
Classify cach compound as either ionic molecular covalent or an acid and then provide its formula Classification Ionic Name Molecular or Acid Formula Calcium phosphide Iron II sulfite Nitrogen tribromide Magnesium nitride Xenon tetroxide Perbromic acid Copper 1 oxide Dinitrogen monoxide Sulfur difluoride Tin IV sulfide lodine pentachloride. Covalent compounds are composed of both non-metals. Whereas a compound in which there occurs transfer of electrons from one atom to another is known as an ionic compound.
Hence it will form compound which is covalent in nature. NH42C104 What is the formula for potassium sulfate. Identify the phrases that generally apply to molecular compounds.
Neon has an octet of electrons. Formula Classification Ionic Molecular or Acid Name CCL FeBros HClaq PF CaCO AIO H SOaq NiC2H022 SO MnO NHOH CF4 Na s HCHO2aq MgOH CrBr. Values from Table 12 below to determine if the following bonds are likely to be non-polar covalent polar covalent or ionic.
5 Classify each of the following as having mainly ionic or. Ionic Compounds When an element composed of atoms that readily lose electrons a metal reacts with an element composed of atoms that readily gain electrons a nonmetal a transfer of electrons usually occurs producing ions. For the polar covalent compounds indicate the direction of the dipole.
Phase Changes And Behaviour Of Gases U4 Toxins. For example atomic number of magnesium is 12 and atomic number of chlorine is 17. Example of some compounds that have multiple oxidation states.
Classify the following substances as ionic compounds or covalent compounds containing discrete molecules. Classify each of the following compounds as ionic or covalent. B lonic ionic covalent.
___ ions are cations or anions that contains more than one atom. H-CI H3C-CHCH3 HCO-H Na-Br H-OH HC-CF H-CH Br-Br F-CI HC-OH Br-Mg -Br Li-CHCH3 2 Determine the formal charge on each of the bold atoms below. MathrmCH_4 mathrmKF mathrmCO mathrmSiCl_4 mathrmBaCl_2 Answer View Answer.
C Covalent covalent ionic. NO3- SO42- OH- NH4 Carbonate Hydrogencarbonate Phosphate CO32- HCO3- PO43- 4 Work out the formulae of the following ionic compounds. Whereas ionic compounds are usually formed when a metal and a nonmetal combine covalent compounds are usually formed by a combination of nonmetals.
So in order to attain stability Mg donates its valence electrons to chlorine atoms which will. Ionic Tripotassiu m Prospnate KaPO ionic Huycrogen Bromide HBr Ocid Salium hudroyide. A CaCl2B CO2C H20D H2So4E Baso4F.
CaCl 2 IONIC 11. Classify the following compounds as IONIC metal nonmetal COVALENT nonmetal nonmetal or BOTH compound containing a polyatomic ion. View Available Hint s Reset Help lithium fluoride sulfur dioxide calcium fluoride strontium chloride nitrogen dioxide aluminum chloride Covalent bonds lonic bonds.
Match each bond to its appropriate classification. Stoichiometry Solution Chemistry And Acids And Bases U5 Fire. Which of the following atoms below hashave five valence electrons.
Bare often gases or liquids. Classify each of the following compounds as ionic or covalent. When both ionic and covalent bonding occurs in a compound.
Energy Thermodynamics And. Explain why neon is monatomic but chlorine is diatomic. Under normal conditions molecular compounds often exist as gases low-boiling liquids and low-melting solids although many important exceptions exist.
Have low melting points. So Hg 2 Cl 2 it the lowest whole number ratio of cation to anion. Molecular Structure And Properties U3 Weather.
B c and e. 6 rows Many compounds especially those discussed in general chemistry courses are classified as either. ___ are negatively charged ions with more electrons than protons.
D lonic covalent ionic. Determine the molecular. Compounds are classified as ionic or molecular covalent on the basis of the bonds present in them.
Complete the table below. Ionic compounds are compounds composed of a metal as the cation positive charge and non-metals as anion negative charge. The difference between ionic and covalent bonds is a bit ambiguous since the only truly nonpolar covalent bond occurs when two elements of the same atom bond with each other eg H 2 O 3Its probably better to think of chemical bonds as being more-covalent or more-polar along a continuum.
Complete the table below. In this respect a water is covalent b covalent c ionic d ionic e covalent f ionic. Part B Classify the following compounds as having covalent or ionic bonds.
Note mercury 1 is not a monoatomic cation but is really a homonuclear diatomic ion of two mercury atoms bound to each other both having lost one electron. Lone pairs have been drawn in for you. Classify the following compounds as ionic or covalent.
Classify each compound as either ionic molecular covalent or an acid and then provide its name. ___ ion is an example of a polyatomic cation. Consider the molecular formulas below and classify the following compounds as ionic covalent or a mixture of ionic and covalent.
Matter Atomic Structure And Bonding U2 Smells. Classify The Following Compounds As Ionic Or Covalent Or Both. ___ are discrete groups of atoms that are held together by covalent bonds.
A lonic covalent covalent. 1 Classify each bond below as ionic non-polar covalent or polar covalent. Then name the compounds.
Contain metals and nonmetals. Classify the following compounds as ionic metalnonmetalcovalent nonmetalnonmetalor both compound containing a polyatomic ion.

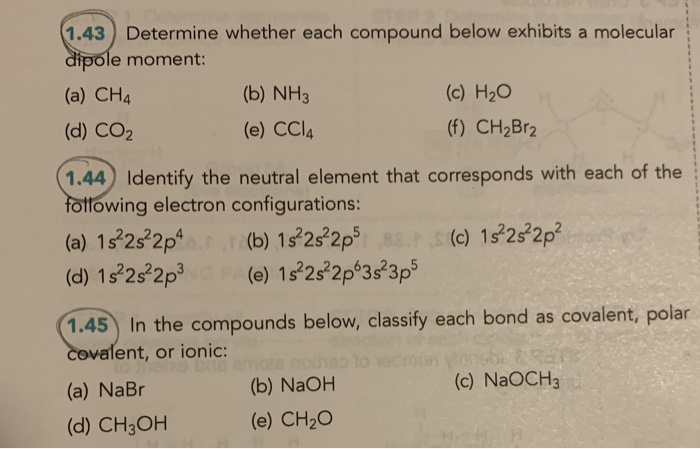
Solved 1 43 Determine Whether Each Compound Below Exhibits Chegg Com
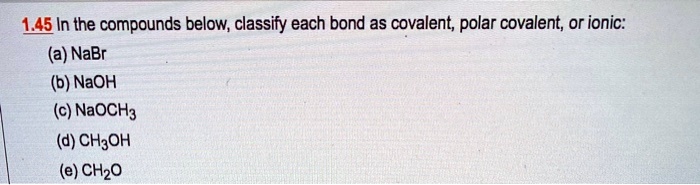
Solved 145 In The Compounds Below Classify Each Bond As Covalent Polar Covalent Or Ionic A Nabr B Naoh C Naoch3 D Ch3oh E Chzo

Solved 1 45 In The Compounds Below Classify Each Bond As Chegg Com
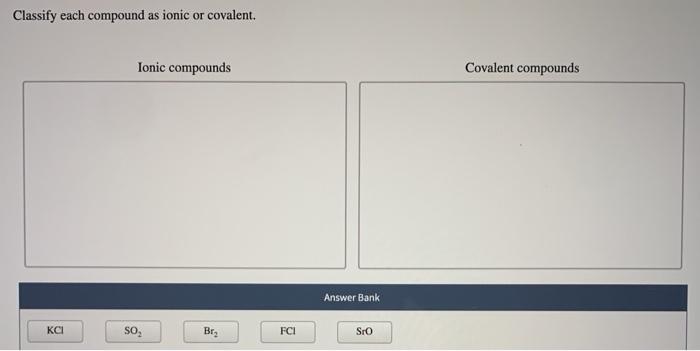
Solved Classify Each Compound As Ionic Or Covalent Ionic Chegg Com
Belum ada Komentar untuk "Classify Each Compound Below as Ionic or Covalent"
Posting Komentar